
 |
|
2 Department of Physical Chemistry, Greifswald University, Soldtmannstraße 23, 17489 Greifswald
3 Department of Geological Sciences, Greifswald University, Jahn-Straße 17a, 17487 Greifswald
| STRUCTURE | |
| Abstract | 4. Conclusions |
| 2. Introduction | Acknowledgements |
| 2. Experimental | References |
| 3. Results and discussion | |
| FIGURES & TABLES | ||
Fig. 1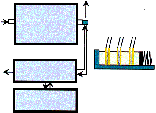 |
Fig. 2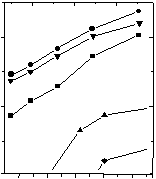 |
Fig. 3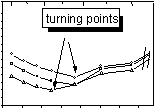 |
Fig. 4a |
Fig. 4b |
|
Tab. 1 |
Tab. 2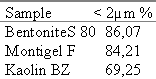 |
Tab. 3 |
Some papers discussing the application of electrochemical properties of clay minerals have already been published. Boutehala & Tedjar (1993) discuss the use of ionexchanged montmorillonite as electrolyte for all solid batteries and all solid pH-sensors. Fitch (1990), Wielgos & Fitch (1993) and Stein & Fitch (1995) have done extensive research on the field of clay modified electrodes.
Some publications are found dealing with the conductivity of clay minerals at lower temperatures. An increase of log (s) with water contents of the clay or with relative humidity is often reported (Fripiat et al. 1965; Calvet & Mamy 1971; García & Bazán 1996; Fan & Wu 1997). García & Bazán (1996) found linear dependence of log(F) with water partial pressure for Na+- and Li+- montmorillonite at 25 °C, divided into two linear regions with different slopes. Linear behaviour was also reported by Fan & Wu (1997) for Na+- and Li+-montmorillonite, they did however not report two separate linear regions. Only little information is found discussing the conductivity behaviour of clay minerals at higher temperatures. Zhu, Wang & Yu (1989) reported a decrease in conductivity with temperature from 90°C up to about 200°C, then an increase up to 610°C.
As to the mechanism of conductivity observed in clay minerals, usually protons are considered the active charge carrying species (Calvet & Mamy 1971; Boutehala & Tedjar 1993). On the other hand, Fan & Wu (1997) emphasize the determining role that interlayer cations play in the conduction process and doubt the main role of protons. Zhu, Wang & Yu (1989) expect a combined conductivity of protons and interlayer cations.
In this article the results of electrochemical research on several natural clay samples will be presented and discussed. In our work we focussed on impedance spectroscopy measurements (IS) as an instrument to investigate the change of electrochemical properties of clays and clay minerals with temerature and gas phase composition. For our experiments we used Kaolin BZ, mainly consisting of kaolinite (a 1:1 double layer clay mineral with uncharged sheets), Bentonite S 80 and Montigel F mainly consisting of Na+-montmorillonite (a 2:1 triple layer clay mineral, with charged sheets and the ability to swell in water or other polar liquids), Glauconite (a 2:1 triple layer mica, with even higher sheet charges and only slight swelling ability in water) and Friedland Tonmehl, mainly consisting of Montmorillonite/Muscovite mixed layer clay mineral.
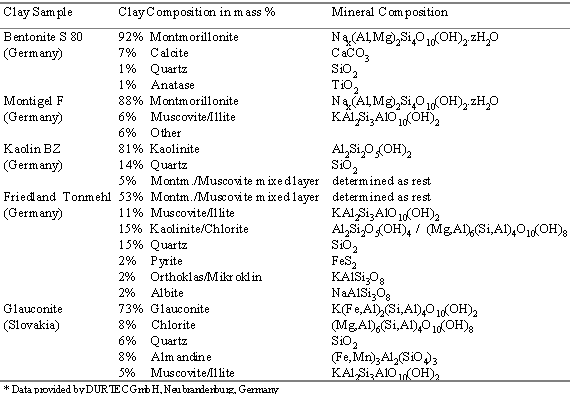 |
Table 1: Mineral compositions of the clay samples under investigation* |
 |
Table 2: Particle size distributions of the clay samples under investigation* |
A defined gas atmosphere was created by leading synthetic gas (20.5% O2 in N2) through a temperature controlled bottle filled with distilled water. For the methanol experiments the main gas stream was split and led through two separate temperature controlled bottles, one filled with methanol and one with distilled water. (For the temperature control two cryostates were used). The gas stream was then led through the quartz tube at a flow rate of 50 ml/min.
The impedance measurements were made by either an EG&G Instruments Potentiostat/Galvanostat Model 283 together with a Solartron Instruments SI 1260 Impedance/Gain-Phase Analyzer or an EG&G Princeton Applied Research Potentiostat/Galvanostat Model 263 together with an EG&G Princeton Applied Research Model 5210 Lock-In Amplifier.
For the measurements a current with an amplitude of maximum 20 mV was used. The frequency ranges lay between 5 MHz and 100 mHz.
For all clay samples the conductivity at 30 °C in dependence of water partial pressure was determined. For Montigel F, Bentonite S 80 and Friedland Tonmehl the conductivity as a function of temperature in the ranges 30 °C up to 70 °C and 150 °C up to 300 °C was determined isothermally. For Glauconite and Kaolin BZ the influence of changing methanol partial pressure at constant water partial pressure was studied at 30 °C.
Equilibrium conductivity values were calculated from the intercept with
the Z’-axis of the Nyquist plot and the geometry factor of the clay pellet.
The system was considered to be in equilibrium if no noticeable change
in impedance behaviour occured during a period of 24 hours.
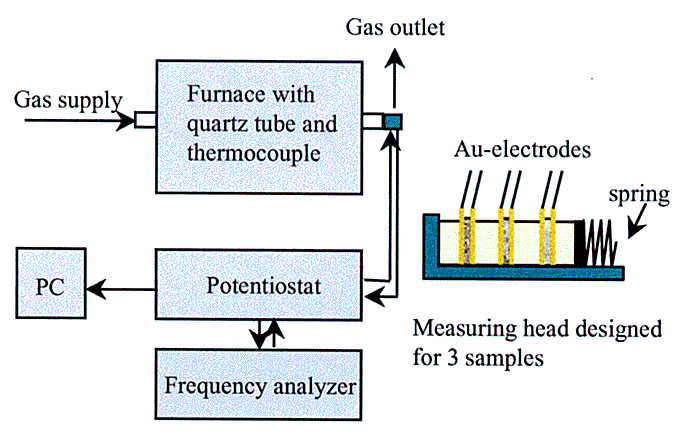 |
Figure 1: Experimental setup and measuring headTop Menü |
Bentonite S 80 and Montigel F show very high conductivity values of over 10-4 S·cm-1 in the entire measured range. Using the classification as given by Colomban & Novak (1992) proton conductors showing such high conductivities are sometimes qualified as super ionic conductors (SIC). Friedland Tonmehl also shows very high conductivity values.
Conductivity data for Glauconite are considerably lower than those for Montigel F, Bentonite S 80 and Friedland Tonmehl. Two linear regions can be identified, the turning point lying by a relative humidity (R.H.) of approximately 0.5. A simular behaviour was reported by García & Bazán (1996) for Na+- and Li+-montmorillonite.
Due to the basic electroneutrality of the sheets the conductivity data
for Kaolin BZ are significantly lower than those for the other samples.
Lattice imperfections, especially on grain edges, are the most probable
explanation for the fact that Kaolin BZ establishes some conductivity at
30 °C. Without such imperfections strongly isolating behaviour would
be expected. Lattice imperfections are also responsible for the fact that
some cation exchange capacity is found in kaolinites
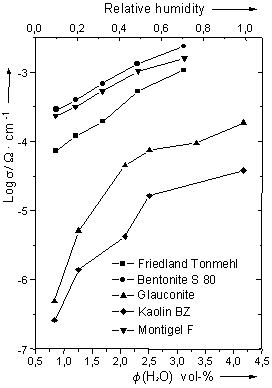 |
Figure 2: Conductivity
data for clay samples in synthetic air at different water partial pressures
and 30 °C. [f (H2O) = F
(H2O)] |
In the range of low temperatures (30 °C - 70 °C), log(F) decreases with temperature. A non-linear behaviour was observed in this range. Around 50 °C the slopes of the curves change strongly. The decrease in conductivity is most likely caused by desorption of surface bound and interlayer water, thereby decreasing the mobility of the charge carriers within the clay structure. At low temperatures water seems to play a very important role in the conductivity mechanism. From fig. 3 can be concluded that in the low temperature region all of the investigated clay minerals have a temperature-dependent negative activation energy.
In the range of high temperatures (150 °C - 300 °C) a further decrease in conductivity was observed, until a turning point is reached. An increase of conductivity with temperature was seen from approximately 210 °C for Montigel F and Bentonite S 80 and 240 °C for Friedland Tonmehl. A simular behaviour has been reported for Mg2+-montmorillonite by Zhu, Wang & Yu (1996).
An estimation of so and Ea
was made for the range between the turning point and 300 °C. The results
of this estimation are given in table 3.
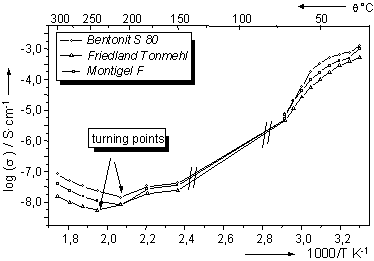 |
Figure 3: Conductivity data for clay samples in synthetic air at different water partial pressures and 30 °C |
 |
Table 3: Estimated Ea and s0 for the clay samples |
From fig. 4 can be seen that the adding of methanol to the measuring gas causes the clays conductivity to decrease.
An explanation for the observed behaviour might be the partial replacement
of water molecules from the structure by methanol, causing the framework
for proton transfer in the clay structure to break down and the solvation
and thereby the mobility of the interlayer cations to decrease. The fact
that complete replacement of water vapour by methanol vapour causes the
conductivity to break down, shows the important role of water in the conductivity
mechanism of clays at lower temperatures. For Glauconite a measurement
was made in synthetic air containing 5.8 vol.% methanol and no water (not
shown in fig. 4). The intercept with the Z’-axis
reached a value of approximately 600 MW for that case.
For the temperature range of 30 °C up to 70 °C a decrease of conductivity of Bentonite S 80, Montigel F and Friedland Tonmehl was observed, the conductivity decreasing very sharply at temperatures > 50 °C. For the temperature range 150 °C up to 300 °C two regions could be observed:
(1) A decrease with temperature until a turning point is reached (210 °C for Montigel F and Bentonite S 80, 240 °C for Friedland Tonmehl).The addition of methanol to synthetic air with constant water vapour concentration led to a decrease in conductivity for Glauconite and Kaolin BZ at 30 °C, the conductivity decreasing with increasing methanol concentration.(2) An increase of conductivity with temperature between the turning point and 300 °C. In that range the curves showed Arrhenius behaviour and Ea and so could be determined.
The results of this work show that impedance spectroscopy can be a valuable tool in characterizing clay minerals and that clay minerals may be interesting sensor materials for the detection of polar substances.
CALVET, R., MAMY, J. (1971): Sur la nature des charges responsables de la conductivité électrique des argiles.- C.R. Acad. Sc. Paris, t. 273 Série D, 1251-1253.
COLOMBAN, P., NOVAK, A. (1992): P.Colomban (ed) ´Proton Conductors: Solids, membranes and gels-materials and devices’.- Cambridge University Press, 38-60
FAN, Y., WU, H. (1997): A new family of fast ion conductor-montmorillonites.- State Ionics 93, 347-354.
FITCH, A. (1990): Clay-modified electrodes: A review.- Clays and Clay Minerals 38(4), 391-400.
FRIPIAT, J. J., JELLI, A., PONCELET, G., ANDRE, J. (1965): J. of Phys. Chem. 69, 2185.
GARCÍA, N. J., BAZÁN, J. C. (1996): Conductivity in Na+- and Li+-montmorillonite as a function of equilibrium humidity.- Solid State Ionics 92, 139-143.
GUTH, U., BROSDA, S., SCHOMBURG, J. (1996): Applications of clay minerals in sensor techniques.- Applied Clay Science 11, 229-236.
STEIN, J. A., FITCH, A. (1995): Computerized system for dual-electrode multisweep cyclic voltammmetry for use in clay-modified electrode studies.- Analytical Chemistry 67(8), 1322-1325.
WIELGOS, T., FITCH, A. (1993): A clay modified electrode for ion-exchange voltammetry.- Electroanalysis 2, 449-454.
ZHU, B., WANG, D., YU, W. (1989): The study of structure and electrical properties of montmorillonite solid electrolyte.- Solid State Ionics 36, 15-22